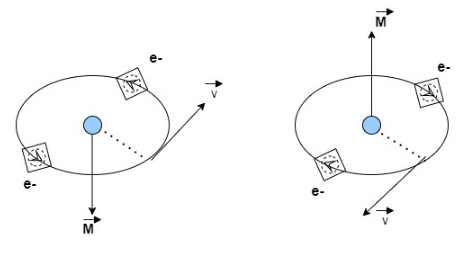
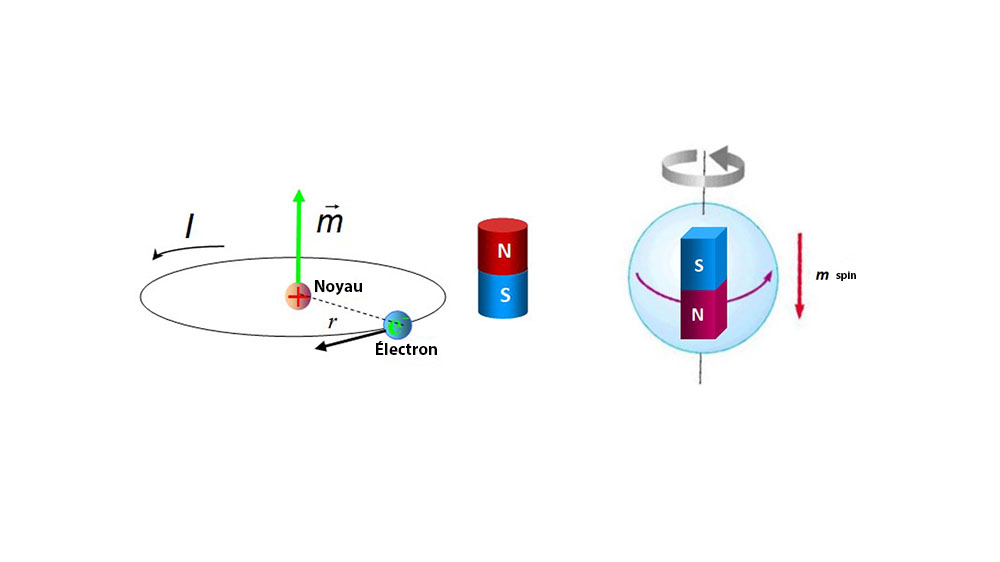
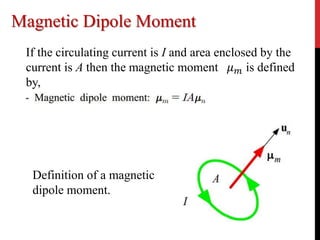
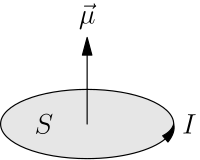
What is the magnetic moment, and what does it have to do with the spin of the electron? - Physics Stack Exchange

THE TOTAL SPIN AND MAGNETIC MOMENT FOR THE ATOM WITH ATOMIC NUMBER 7 ARE ? PLZ EXPLAIN THIS QUESTION IN DETAIL . IF YOU LIKE MY QUESTION THEN DON'T FORGET TO UPVOTE

In a hydrogen atom, the binding energy of the electron in the ground state is E1. Then the frequency of revolution of nth electron in the nth orbits is

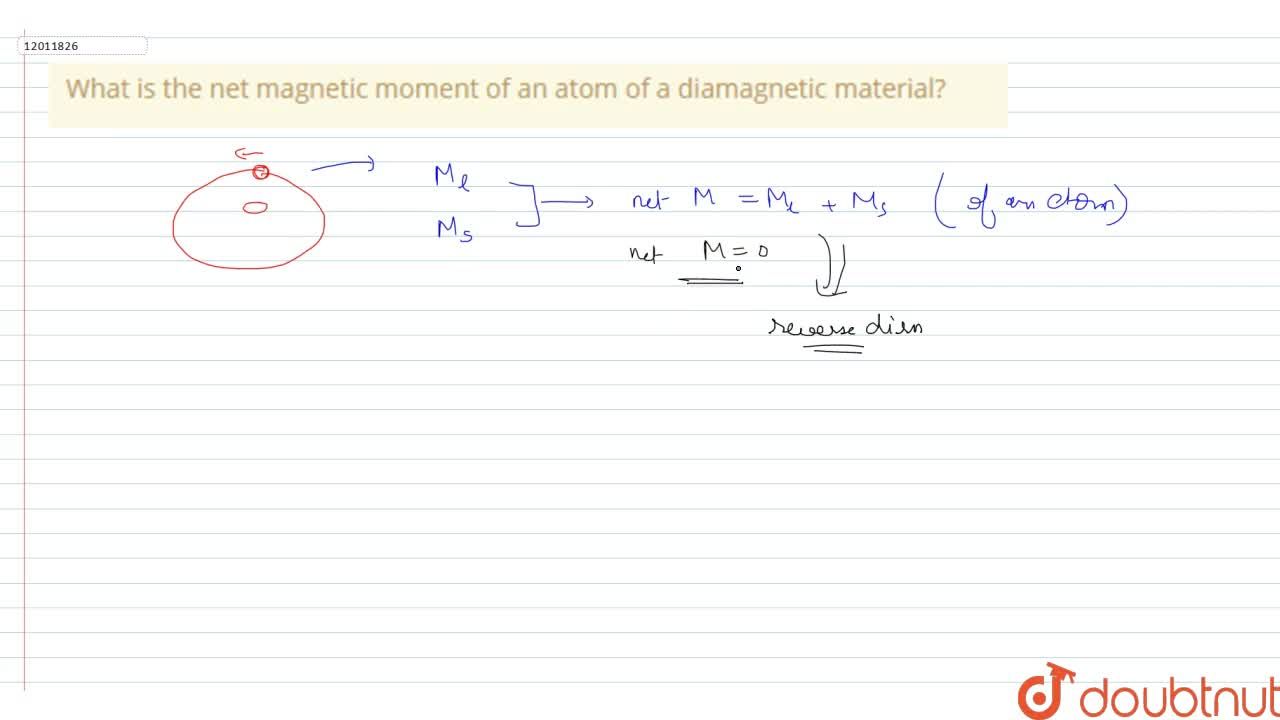
SOLVED:The magnetic moment of a diamagnetic atom is : (a) zero (b) infinity (c) negative infinity (d) another value
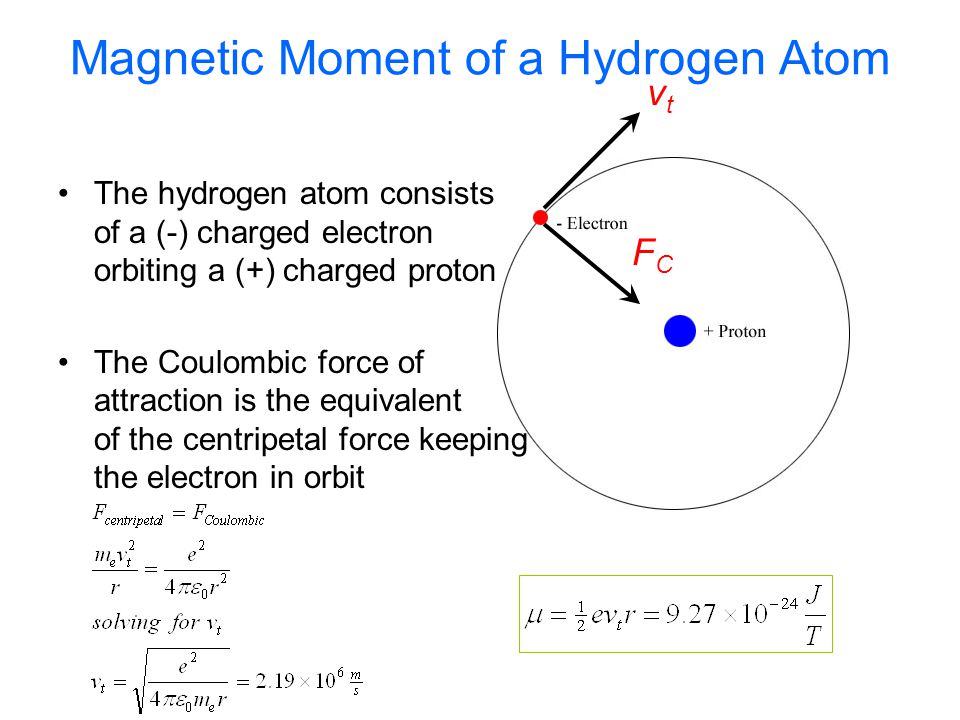
In a hydrogen atom, an electron of charge revolves in an orbit of radius r with speed v. What is the magnitude of the resulting magnetic moment of the electron? - Quora

Physics - Ch 66.5 Quantum Mechanics: The Hydrogen Atom (34 of 78) Magnetic Moment in Hydrogen - YouTube
Calculate the magnetic moment of an atom (in Bohr magnetons) (a) in 1F state; (b) in 2D3/2 state; - Sarthaks eConnect | Largest Online Education Community

Three protons coming from excited atomic hydrogen sample are picked up. Their energies are 12.1 eV, 10.2 eV and 1.9 eV . These photons must come from